BREAST TO BRAIN METASTASIS
Brain metastasis is a fatal complication for patients with cancer.
The incidence of brain metastasis is up to 10-fold higher that that of primary brain cancer. More than 45% of patients with advancer stage triple-negative breast cancer will develop brain metastasis. The lack of effective treatment options confers poor prognosis.
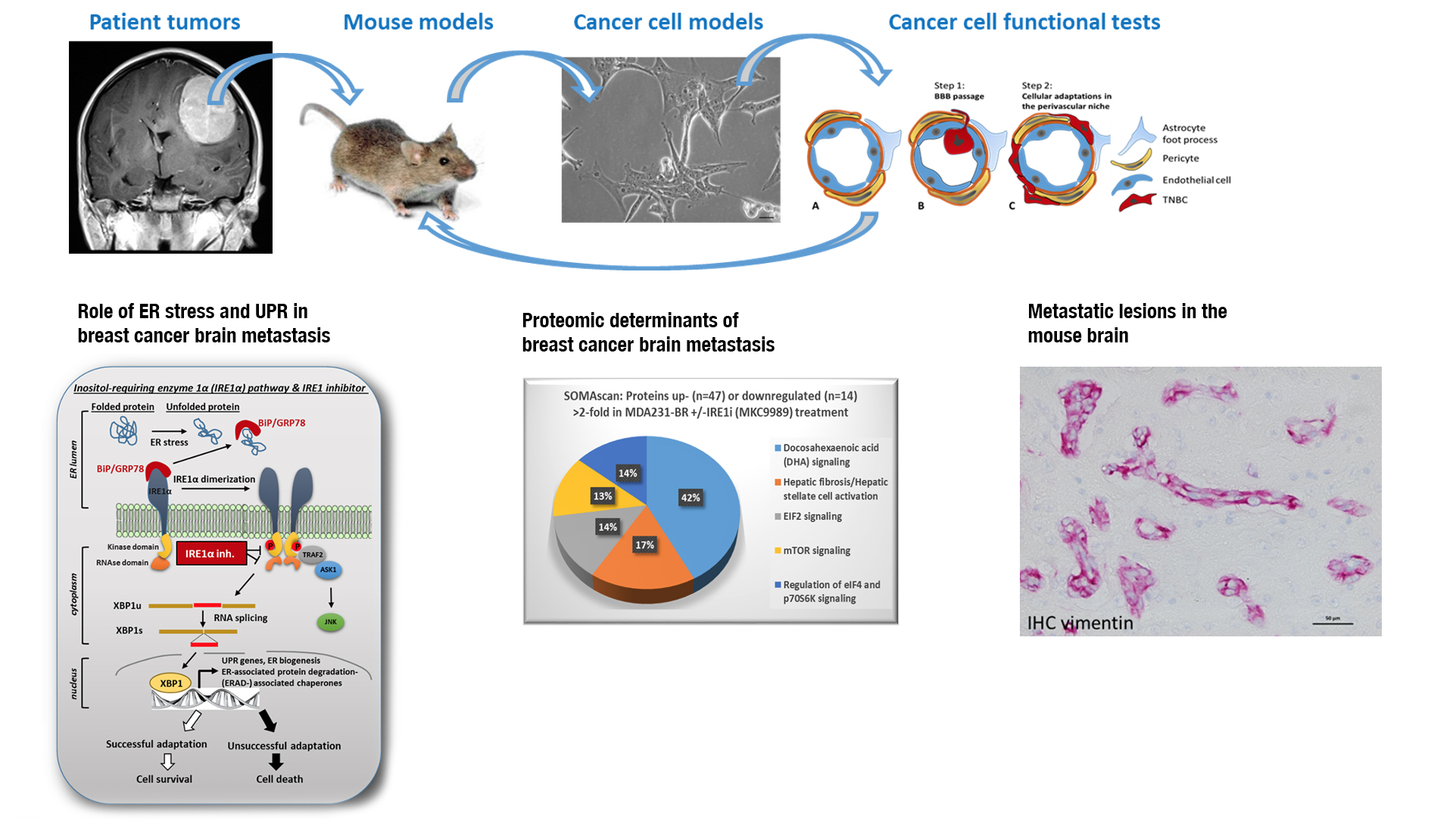
We are interested in studying the cellular mechanisms that facilitate breast cancer cell colonization of the brain and cancer cell survival in the perivascular niche.
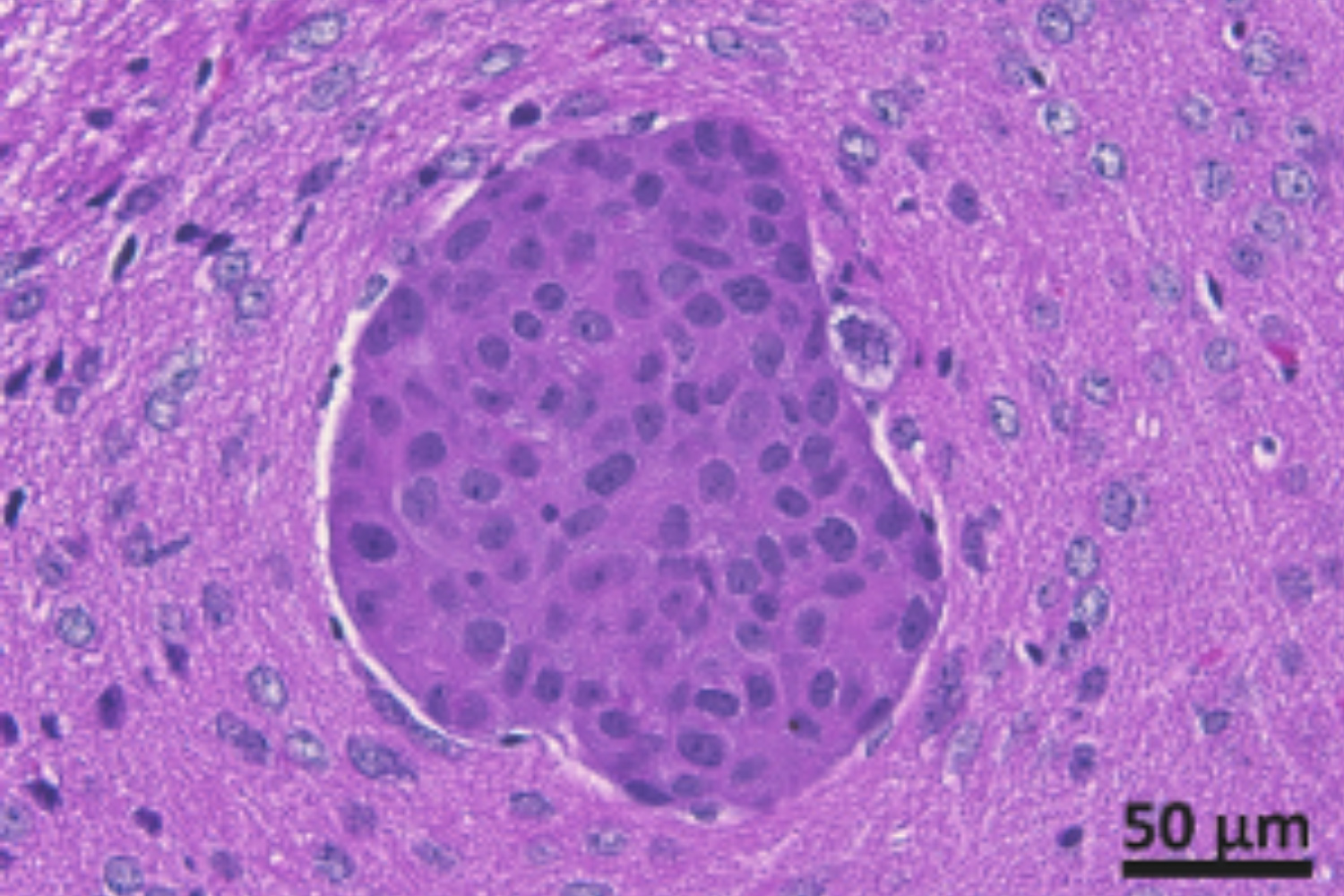
We have isolated metastatic breast cancer cells from surgically removed brain metastatic lesions. Our lab has established mouse models for breast cancer brain metastasis with patient brain metastatic cancer cells to create cell models for brain metastasis research.
Elucidating the paracrine mechanisms and cell-cell interactions between tumor cells and adjacent cells of the perivascular niche will provide important clues for the design of preventative and therapeutic interventions to help cancer patients.
OUR CURRENT RESEARCH FOCUS IS:
Endoplasmic reticulum stress (ER stress) is a cellular stress response. Three transmembrane ER proteins function as ER stress sensors and elicit the unfolded protein response (UPR) as adaptive cellular mechanism to mitigate the ER stress and promote cell survival. We are specifically focusing on the regulation of the Inositol-requiring enzyme 1α (IRE1α) pathway of UPR as a potential facilitator for brain colonization of metastatic cells and their survival in the perivascular niche. Specific inhibition of the IRE1α RNAse activity results in distinct proteomic changes in human breast cancer brain metastasizing cells that may influence their brain metastatic competency.
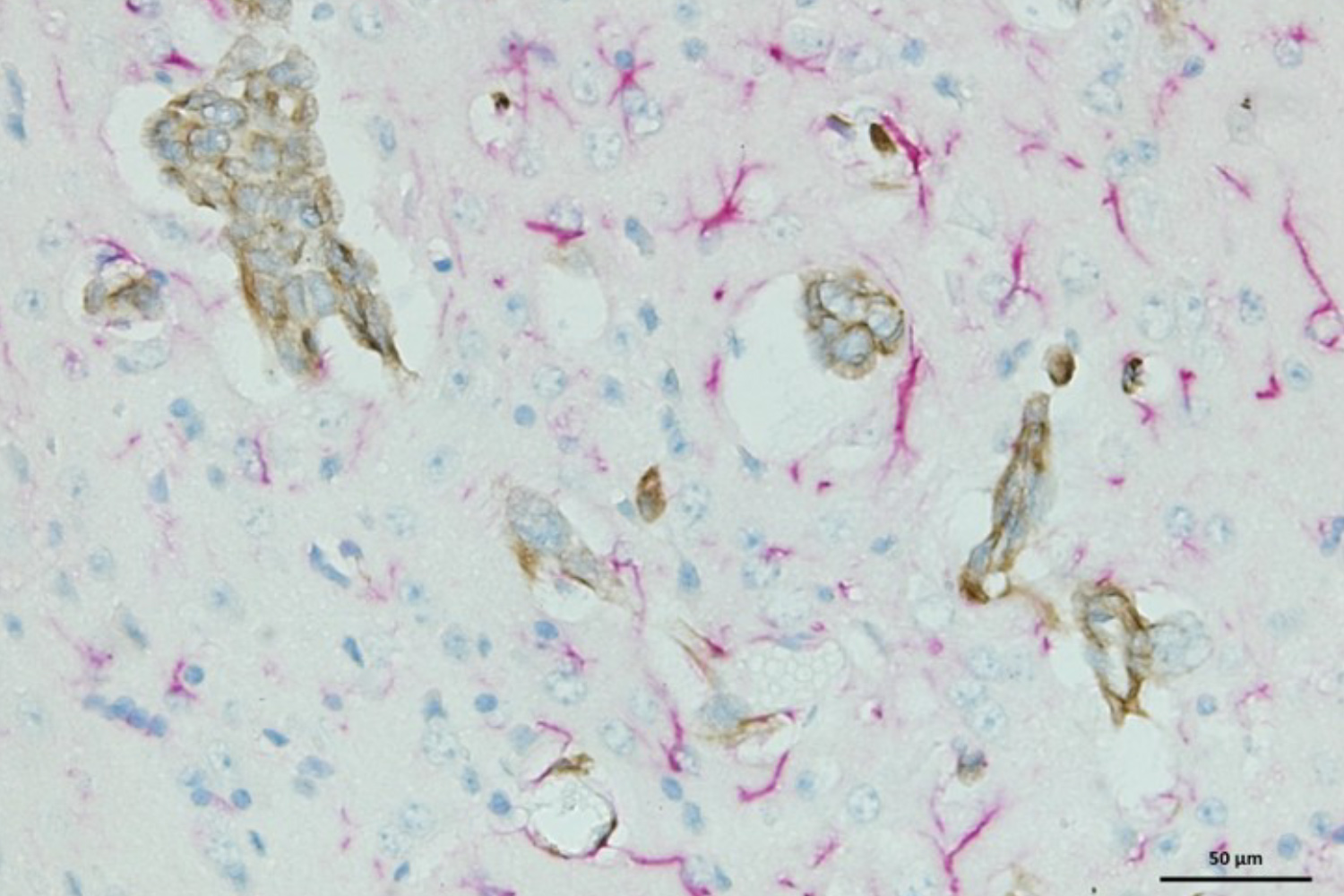
Brain metastasis requires the specific interaction of cancer cells with the endothelium at brain capillaries and post-capillary venules that finally result in the cancer cells crossing of the blood-brain-barrier. Different from other organs, microvessels in the brain have higher than 50% coverage by perivascular pericytes, a cell type with high phenotypic plasticity and stem cell capability. We aim to better understand the cell-cell communication between metastasizing cancer cells and these resident cells of the in the perivascular niche to identify key adaptive mechanisms that are required for survival and early metastatic growth.
We perform proteomic profiling and kinome arrays of patient-derived brain metastatic cells to discover prominent survival pathways that can be targeted by drugs. In collaboration with the NIH we test novel brain penetrable drugs for their effectiveness in preventing brain colonization or delaying metastatic growth in our mouse model for brain metastasis.