HMGA2 IN CANCER
HMGA2 (High-Mobility-Group AT-Hook-2) is expressed in embryonic and adult stem cells and plays a role in growth, adipogenesis and mesenchymal differentiation.
HMGA2 is post-transcriptionally silenced in adult differentiated cells by the microRNA Let7 which binds to the 3’UTR of HMGA2 mRNA leading to its degradation. In the human genetic variants of HMGA2 are associated with stunted growth, Silver-Russel Syndrome, or gigantism.
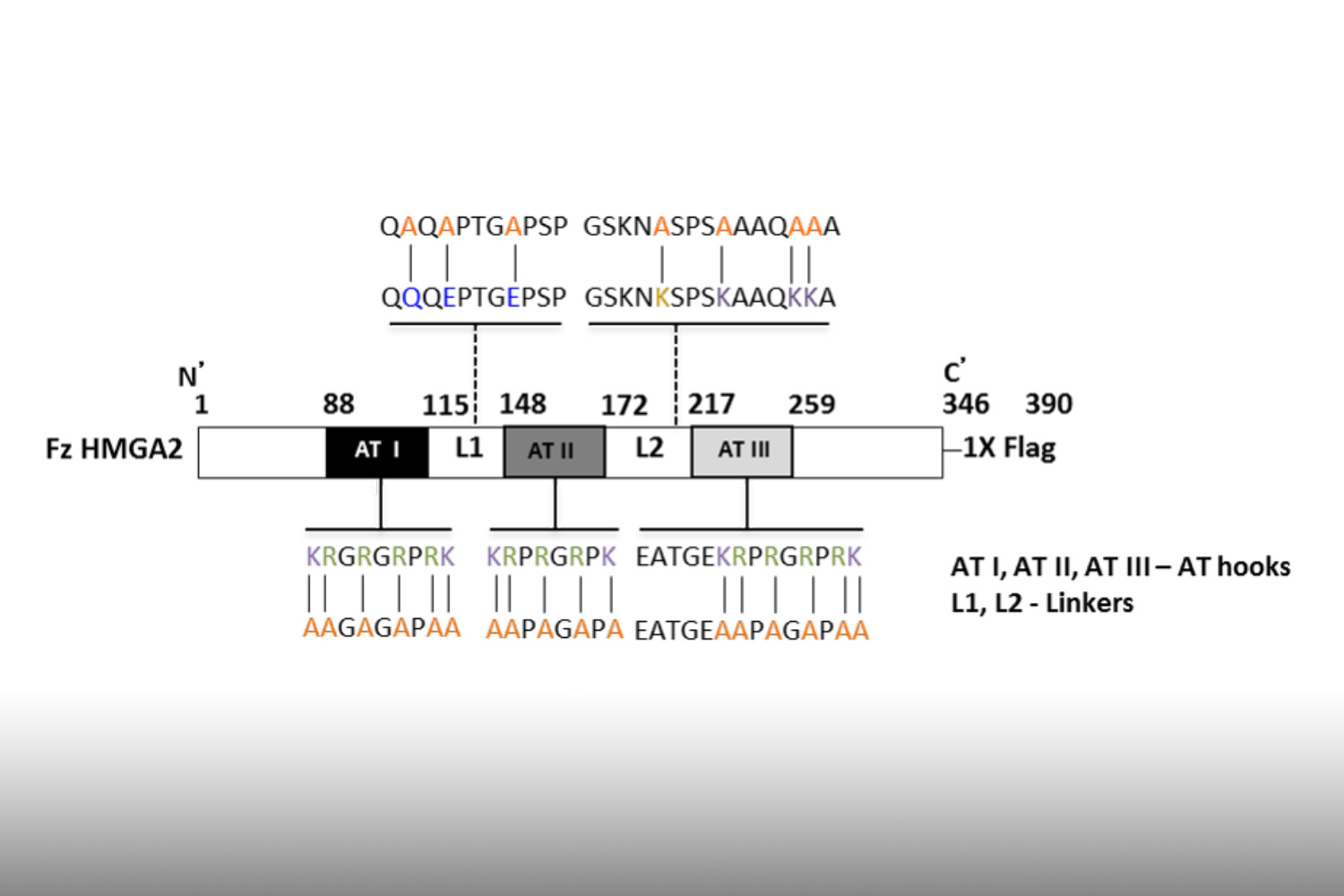
HMGA2 is found to be re-expressed in many cancer cells due to activation of the STAT3/LIN28/Let-7 regulatory pathway, among others, or due to gene fusions and amplifications. DNA-binding occurs via three AT-hook domains and facilitates HMGA2’s functions in chromatin remodeling and as transcriptional regulator in EMT signaling.
We investigate the role of the non-histone chromatin binding protein HMGA2 in cancer cell chemoresistance with a focus on HMGA2 involvement in DNA damage repair and cancer cell survival under chemotherapeutic stress.
MAJOR TOPICS WE ARE CURRENTLY WORKING ON INCLUDE:
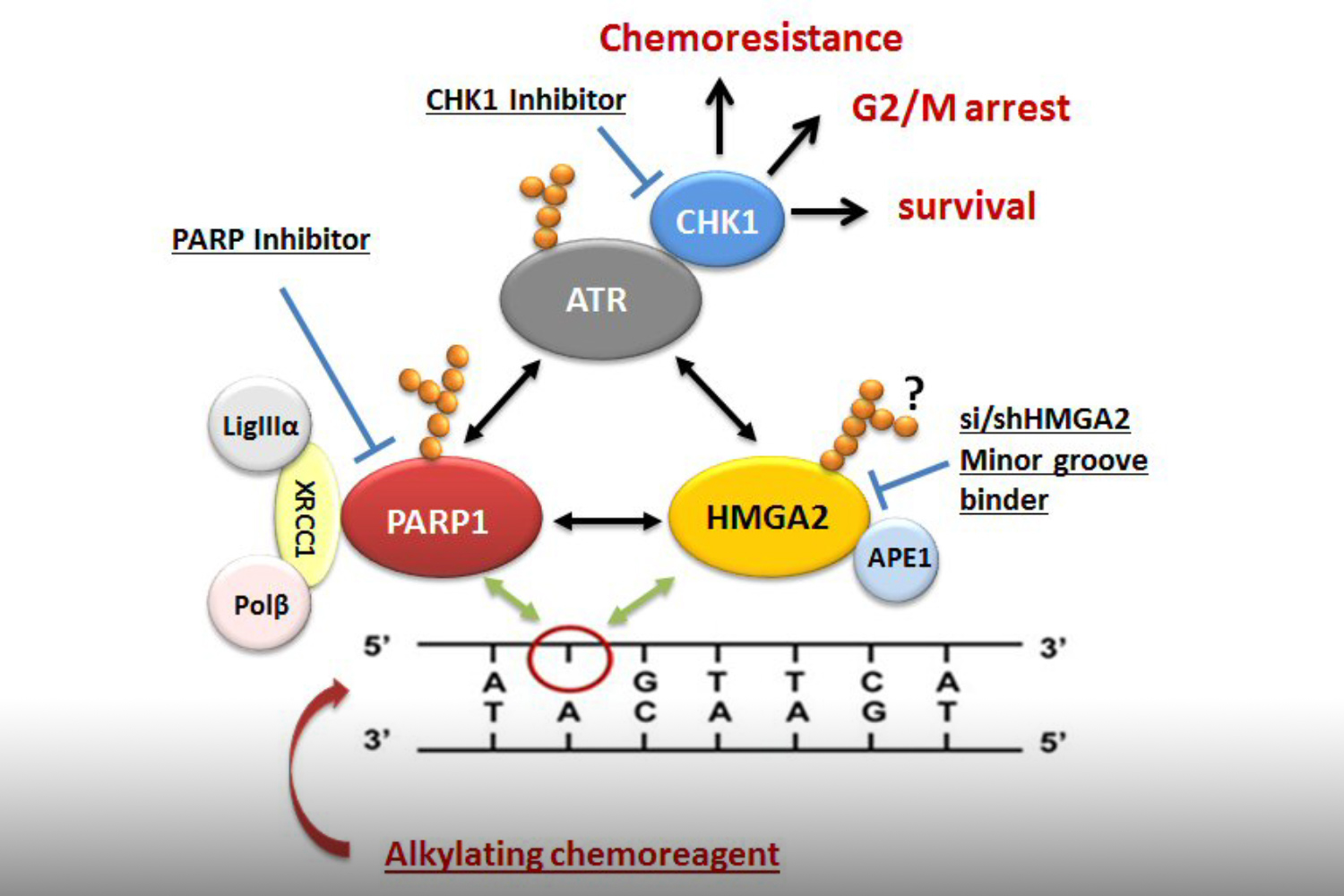
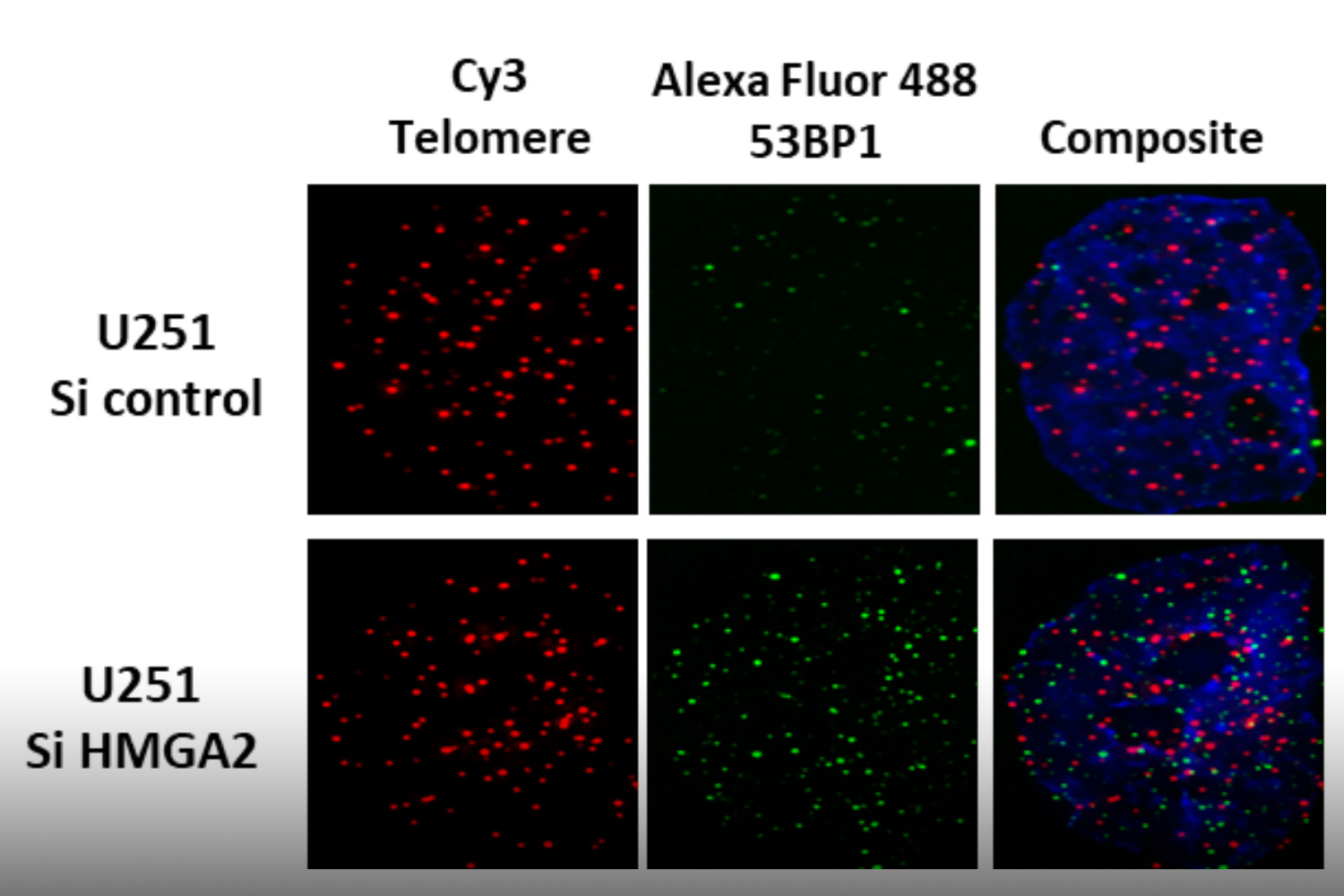
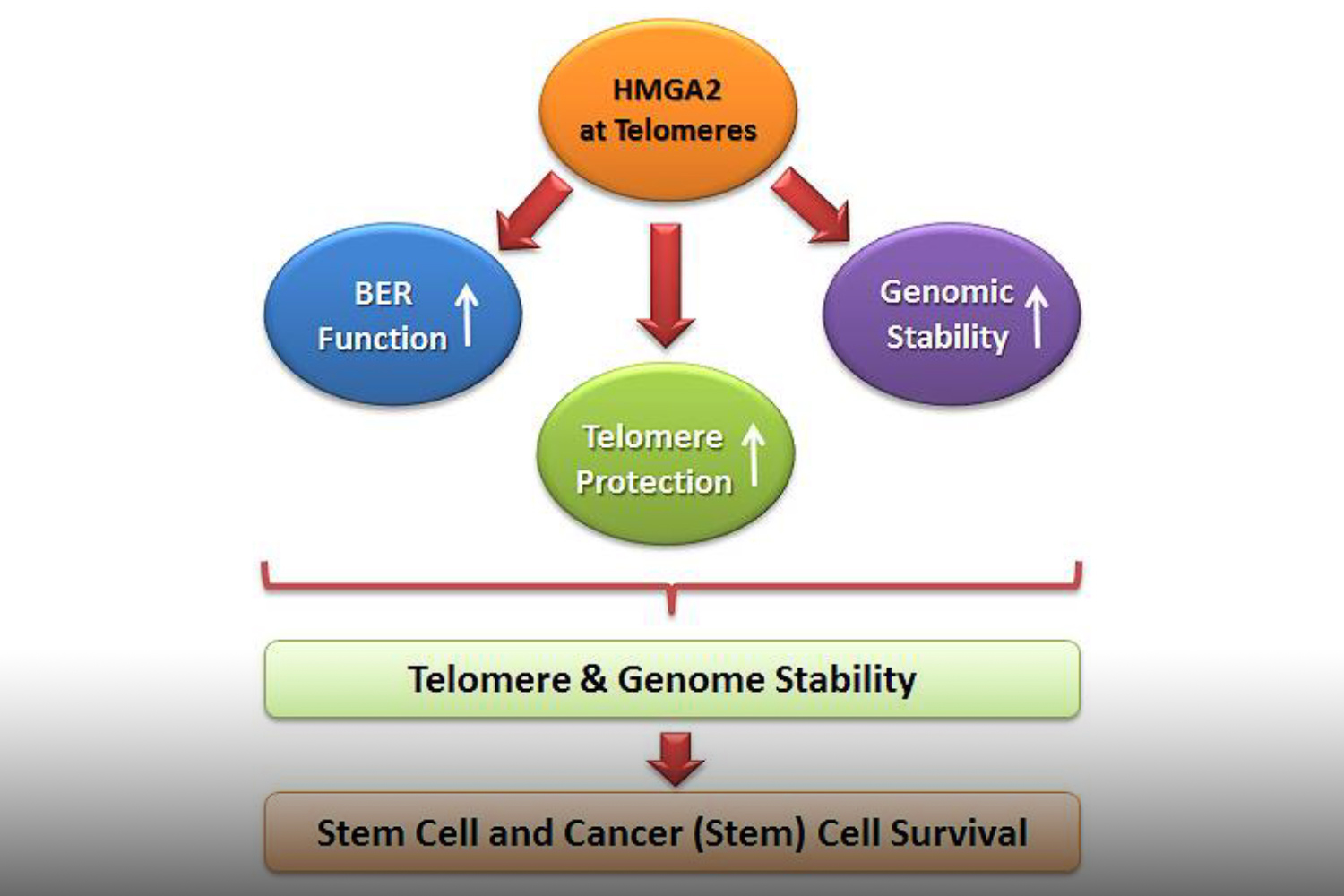
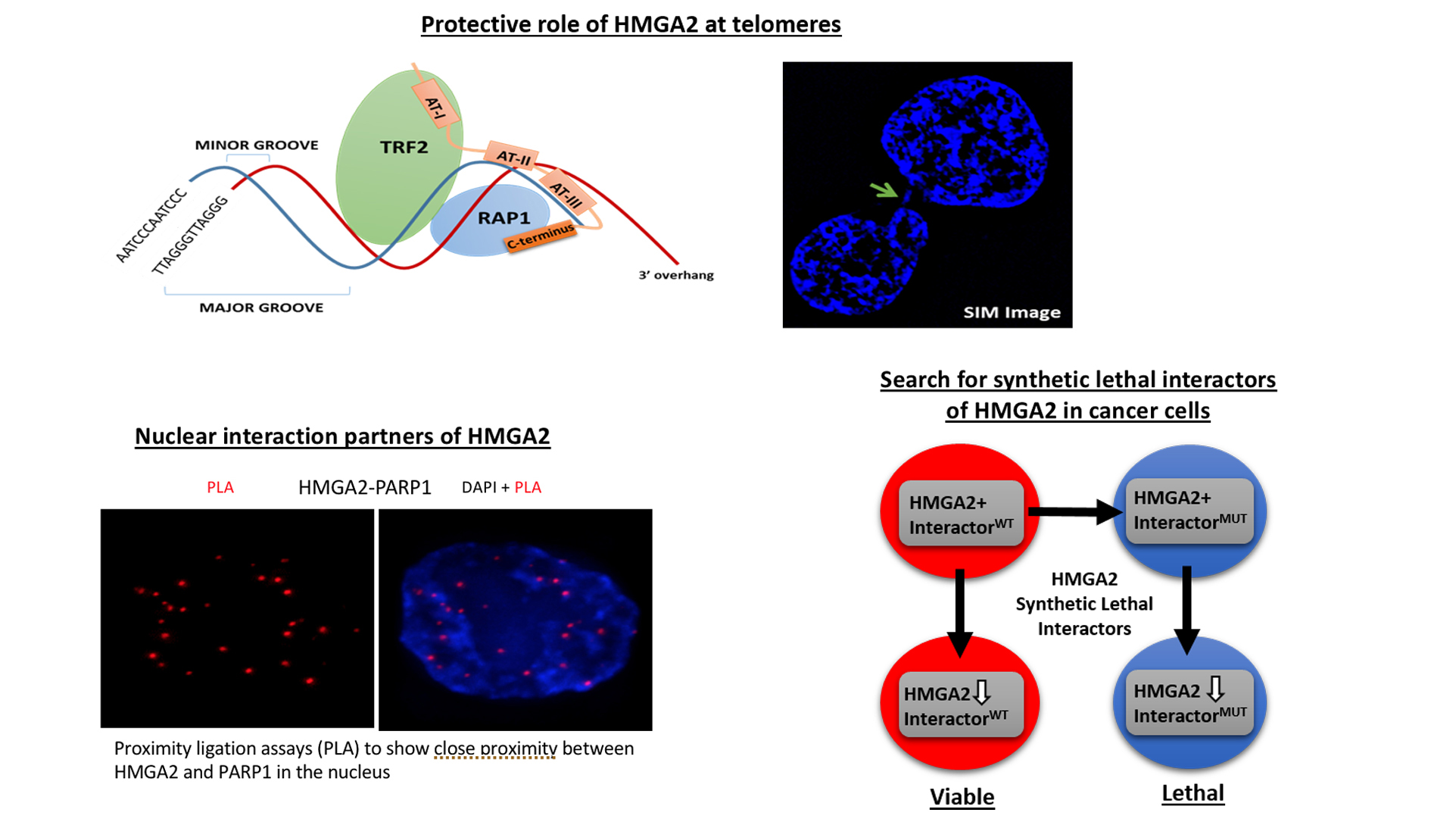
To understand which DNA damage repair pathways are supported by HMGA2 and which mechanisms are involved we determine the HMGA2 protein interactome in the cell nucleus. Identifying novel HMGA2 interacting partners under conditions of oxidative and chemotherapeutic stress will provide HMGA2-supported DNA repair pathways that can be targeted in HMGA2 expressing tumor cells under DNA stress.
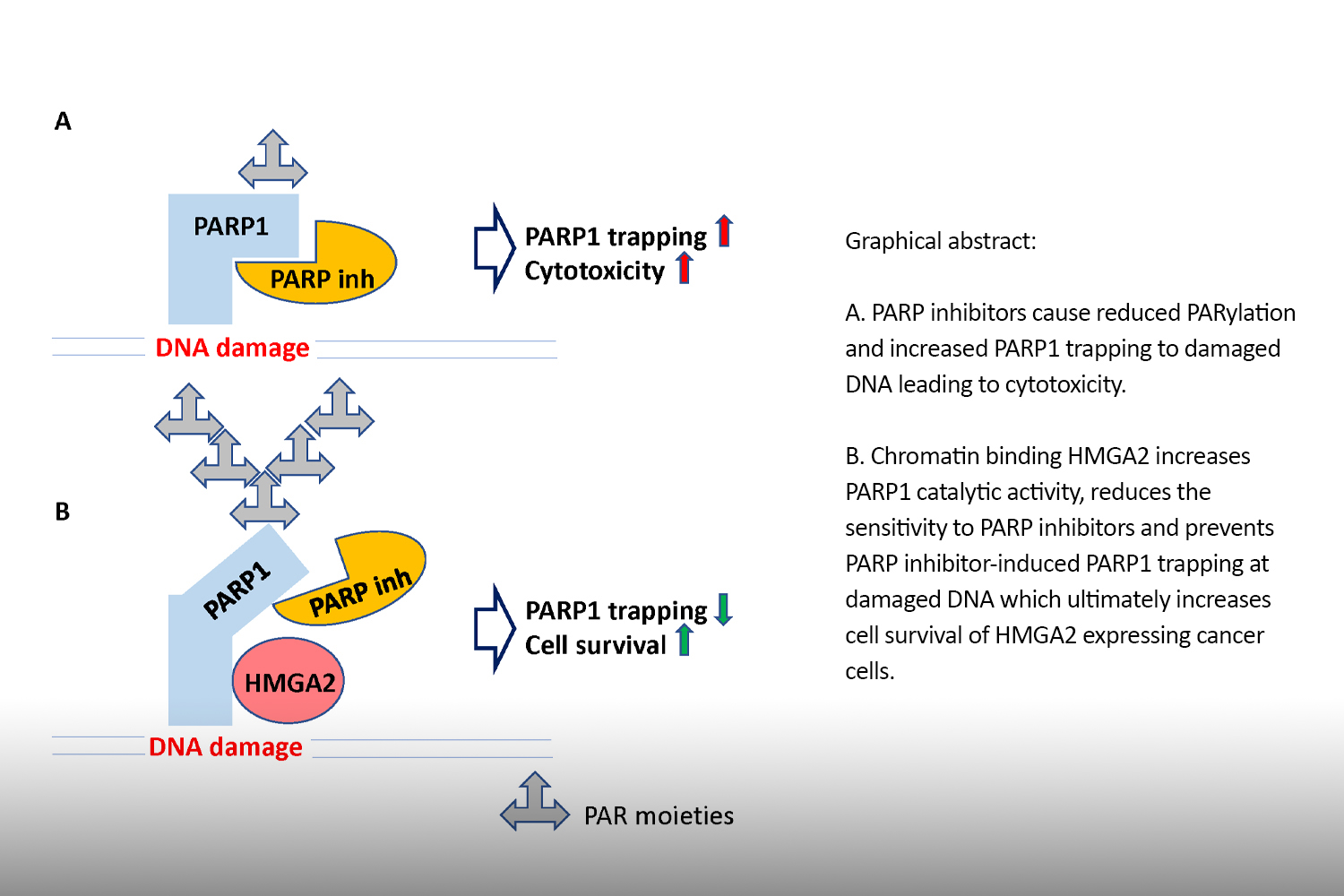
We discovered that HMGA2 binds to PARP1 and increases DNA damage-induced PARP1 activity, a capability that potentially influences several DNA damage repair pathways in cancer cells. We use reporter plasmids for different double strand repair mechanisms to identify HMGA2’s contribution to DNA maintenance. Our lab investigates domain-specific mutants of HMGA2 to define the molecular requirements for the HMGA2-PARP1 interaction and its role in DNA repair and cell survival under DNA stress. We developed mutant HMGA2 cell models and HMGA2 knock-out cell models to study the ability to bind PARP1 and other proteins with functions in DNA damage sensing and repair.
HMGA2 is linked to high invasiveness and poor outcome for patients. Some cancer cells depend on HMGA2 for survival. We search proteins in DNA damage sensing and repair pathways that essentially facilitate cell survival under loss of functional HMGA2. These are synthetic lethal interactors of HMGA2. They inform on essential roles HMGA2 plays in cancer cells and help identify combinatorial treatment strategies that would reduce resistance.